Sinovac, Numolux and Afrivac working on alternative vaccines for Africa
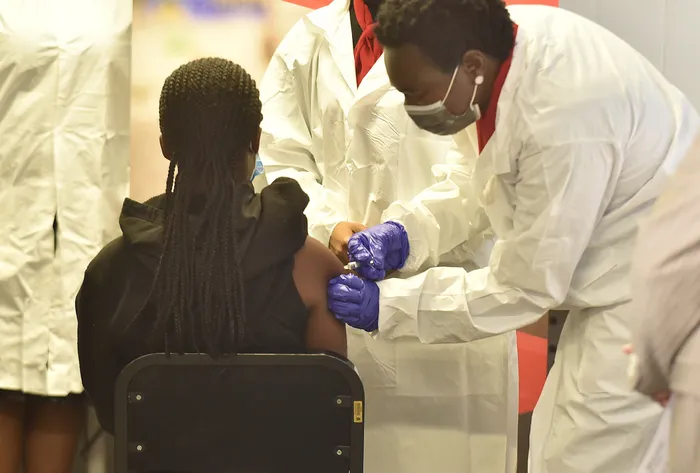
The first girl child is vaccinated at Sefako Makgatho Health Sciences University. Numolux and China’s Sinovac have partnered to conduct a paediatric trial for Covid-19 vaccines for children and adolescents aged between six months and 17 years old. Picture: Thobile Mathonsi/African News Agency (ANA)
Cape Town - The Numolux Group, which has partnered with Sinovac Biotech Ltd, who are moving towards phase 3 of their paediatric trials in South Africa which began in September, said there has been an increase in demand for its CoronaVac vaccine over mRNA vaccines.
Numolux and Sinovac have also partnered on a vaccine manufacturing plant in Saldanha called Afrivac to produce jabs which can help with other vaccines.
This plant will produce the inactivated Covid-19 CoronaVac, as well as other vaccines for illnesses which continue to plague South Africa, and the African continent.
The group along with Sefako Makgatho Health Sciences University and Sinovac made headlines in September when 2 000 children and teenagers between six months and 17 years old formed part of their paediatric trial.
In October, the National Institute for Communicable Diseases (NICD) said the Covid-19 vaccine was available for children between the ages of 12 and 17.
The NICD said: “As of mid-October 2021, children aged 10 to 19 years made up 9.2% of all cases of Covid-19 reported since the beginning of the pandemic.”
The Western Cape Health Department said vaccines reduced severe illness even in children and that by October many of the cases involved children.
“Although Covid-19 in younger persons is sometimes milder than in adults, some youth infected with the coronavirus can get severe lung infections, become very sick and require hospitalisation. The youth can also have complications such as multi-system inflammatory syndrome that may require intensive care or long-lasting symptoms that affect their health and well-being. Therefore, it is important for younger persons to get vaccinated as it is for the vulnerable members.”
Numolux group chairperson and CEO Hilton Klein said they had seen an increase in the demand for their vaccine as opposed to mRNA vaccines.
CoronaVac is produced by using more classic technology, such as those traditional vaccines used to protect South Africans against childhood illnesses.
Klein said what made it different was that people had a choice.
“We are inundated with requests from ordinary South Africans, many who are not comfortable with taking the mRNA vaccines, who want to use an inactivated Covid-19 vaccine like CoronaVac, and we think it is only fair that people have the choice of what type of vaccine enters their body. CoronaVac has received emergency use approval by SAHPRA in July 2021, and we are hopeful that it soon becomes part of the government’s Covid-19 national roll-out of vaccines.”
Kevin Zhang, Sinovac’s senior director of international business, who is currently in South Africa, said Sinovac’s CoronaVac Covid-19 vaccine has been approved in 61 countries and regions, including the World Health Organization and the AU, for either emergency use or conditional approval.
Zhang said that Sinovac has supplied nearly 2.8 billion doses of CoronaVac worldwide, with nearly 2.3 billion doses being administered.
“Sinovac produces a wide range of vaccines and also supplies its other vaccine portfolios, including the hepatitis A vaccine and the flu vaccine, to many other countries,” said Zhang.
Klein said they collaborated with Sinovac to develop vaccines which will be promising for other illnesses as well.
“Numolux and Sinovac have a long-term strategic partnership and relationship, which includes current paediatric clinical trials for Covid-19 in South Africa.
“We also have a partnership on developing vaccines not only for Covid-19, but also for vaccines which can help fight other illnesses which plague South Africa and the continent of Africa. To help in this fight, Numolux and Sinovac have partnered on a local vaccine-manufacturing plant in Saldanha called Afrivac.
“The first phase of this plant has already been completed. This facility will create jobs, as well as increase the capacity of South Africa and Africa to become less dependent on Western countries for not only the Covid -19 vaccine, but other vaccines as well. We need to create vaccines for Africa, by Africa.”
Zhang said the clinical trials for children and adolescents were still under way and will use data to prove efficiency once it has been completed in six countries and that phase 3 was to take place in April.