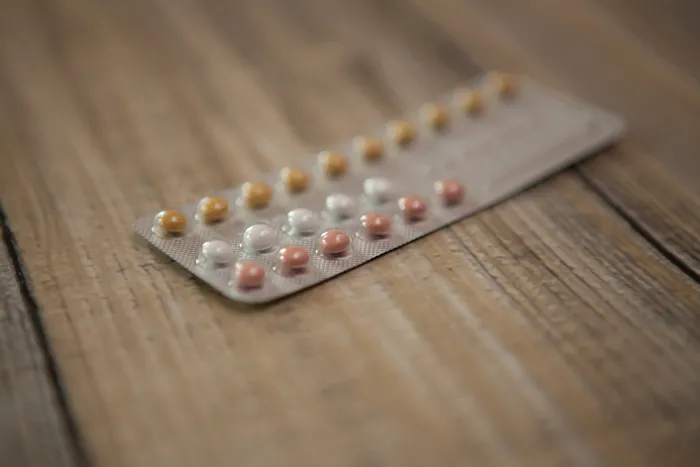
Check your contraceptive packs to see if you qualify to join a lawsuit over a packaging error which resulted in unintended pregnacy.
Image: Pixabay
In a troubling development that highlights the critical importance of effective contraceptive use, LHL Attorneys Inc. is weighing the possibility of a class action lawsuit against Bayer (Pty) Ltd. This consideration follows a substantial packaging error concerning the YAZ PLUS oral contraceptive pills, which has resulted in a wave of unintended pregnancies among women across South Africa.
The issue stems from a specific batch of YAZ PLUS contraceptive pills distributed from November 2023. Identified as batch number WEW96J, these pills featured a dangerous mix-up in their packaging: 24 hormone-free (light orange) tablets were mistakenly included alongside only four active hormone (pink) tablets, rather than the intended reverse of 24 active and four placebo tablets. This packaging mishap, described by the South African Health Products Regulatory Authority (SAHPRA) as one that “drastically undermined contraceptive efficacy,” led to a nationwide Class II recall issued on November 21, 2024.
Angelique Pienaar, a managing associate at LHL Attorneys Inc., did not mince words when addressing the gravity of the situation. “Birth control is essential for exercising your right to bodily autonomy and family planning. Packaging errors, such as including too many placebo pills, compromise its effectiveness, leading to unintended pregnancies, along with significant personal, financial, and emotional consequences,” she asserted in a recent media statement.
The law firm emphasised that under the Consumer Protection Act 68 of 2008, manufacturers can be held accountable for defective products such as these contraceptive pills. This means that women affected by this error may have grounds to seek compensation for a range of damages, including medical expenses, financial losses, emotional distress, and the ongoing responsibilities tied to raising an unintended child.
In a revealing interview with The Mercury, Pienaar shared that the firm has already received information from seven women who experienced unintended pregnancies while using YAZ PLUS during the problematic distribution period. “We expect that more affected users may come forward as awareness grows and as women begin connecting their experiences to the recall notice,” she added, reflecting a growing concern amongst the public.

6549 Illustration of teenage pregnancy. Photographic studio, Johannesburg. 110411 - Picture: Jennifer Bruce Seven women who fell pregnant unintentionally have already contacted the lawyers.
Image: Jennifer Bruce
While the proposed class action specifically focuses on individuals who became pregnant due to the defective packaging, Pienaar encouraged any women who suffered other adverse effects related to YAZ PLUS to reach out to the firm. They will assess each situation to determine the likelihood of additional claims. “No one should have to face the repercussions of a manufacturing error alone,” she noted.
Pienaar explained the procedural steps involved in launching a class action, revealing that it begins with submitting a certification application to the court. This entails a hearing where the court evaluates whether the case should proceed on a collective basis rather than as separate claims from each individual. If successful, the court will issue a summons, instigating proceedings focused on the merits of the case. “The ultimate goal of this process is to ensure that all affected women can pursue justice collectively, efficiently, and fairly, without each person having to carry the burden of individual lawsuits,” she emphasised.
LHL Attorneys Inc. urged women who purchased or used YAZ PLUS between November 9, 2023, and January 31, 2025, and believe they experienced unintended pregnancies, to contact them for a free consultation. Moreover, consumers are encouraged to check their blister packs for batch number “WEW96J,” discuss their concerns with a healthcare provider, and retain any evidence of purchase or use.
As of the time of publication, Bayer has not responded to requests for comment regarding the matter.
Related Topics: